Intracellular interactions between proteins soon to be explained
How do proteins interact within cells? This is a complex question that may be answered thanks to the work of an international consortium led by Jérémie Torrès, Professor the Institute of Electronics and Systems (IES) at the University of Montpellier. Winner of the prestigious European FET-Open call for proposals, this project is based on the development of a biosensor that can directly measure these interactions inside living cells.
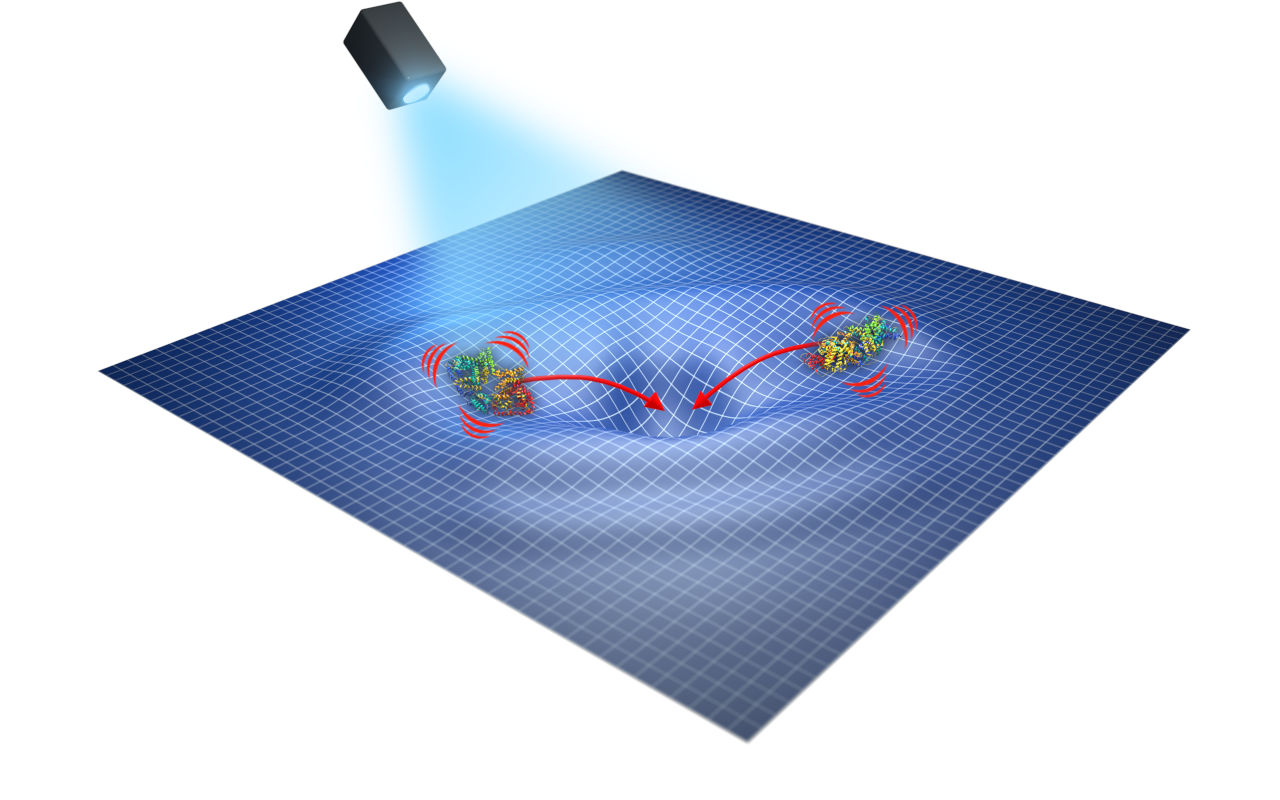
Pour un public averti, cette question ne date pas d’hier. Prédites dès le début du XXème siècle par l’électrodynamique classique et quantique, les forces intermoléculaires agissant à longue distance (<1000 angströms) n’ont jamais été démontrées expérimentalement. Plus pour longtemps, comme l’explique Jérémie Torrès, enseignant-chercheur à l’Institut d’Électronique et des Systèmes (IES) : « Nous avons trouvé un système d’étude qui permet de démontrer ces interactions entre molécules, qui se trouvent être des protéines. »
The "we" used by Jérémie Torres refers to a consortium of four French laboratories under the supervision of the CNRS, including two laboratories in Montpellier (the IES and the Charles Coulomb Laboratory (L2C)), one German laboratory, one Swedish laboratory, and two companies: the Montpellier-based start-up TeraKalis and the Netherlands-based company Micronit. At the instigation of the Montpellier researcher and his colleague at L2C, Sandra Ruffenach, the project was submitted to the prestigious European FET-Open call for research and innovation actions (RIA)... and was selected with the highest score!
Coincidence or design?
It all starts with an observation: when proteins move around in the cytoplasm, they collide with each other and are involved in a very large number of biomolecular reactions in a very short period of time. "It's a bit like when you start a game of pool by breaking, the balls then fly off in all directions at random," explains Jérémie Torres. "This is what we call Brownian motion." Although this observation has long been accepted as dogma, it poses a problem: how can these countless reactions be effective if they are guided only by chance?
This is the problem that Jérémie Torres and his colleagues set out to tackle. "We are not the first. In the 1960s, the English physicist Herbert Fröhlich had already raised it." Biology has already demonstrated how, over short distances (less than ten angstroms), well-known forces in physics influence these intermolecular interactions. "Electrostatic forces electrostatic forces of Coulomb, vvanr Waals, etc., explain how proteins, when they are very close to each other, end up attracting each other and sticking together to initiate reactions. But what we don't know is why they came together. Are there mechanisms that allow them to "call" eachother?asks the IES researcher.
Spectral signature
Thanks to €3.1 million in funding from Europe as part of the FET-Open program, researchers will be able to work on developing a new technology for measuring these interactions. "We are working on a biosensor prototype that will enable us to measure these interactions using the terahertz spectral signature of proteins in an aqueous environment. We are currently the only ones in the world with this expertise."
Since each protein has its own unique spectral signature, this could be used to identify it non-invasively. To measure it, researchers will, in a sense, force the proteins to synchronize their oscillations, "like metronomes that are forced to oscillate at the same time," adds Jérémie Torrès. "When proteins oscillate collectively, they resonate at a specific frequency that falls within the terahertz range. We will measure these resonances to highlight the long-range intermolecular interactions that could be responsible for the mechanisms governing molecular dynamics within cells."
Physical sciences vs. science fiction
While this research project is primarily focused on fundamental and technological aspects—better understanding the mechanisms of attraction between proteins—it could also, in the long term, lead to major therapeutic innovations. How? By acting on these mechanisms, or even inhibiting them, when they are involved in the development of certain diseases. "The dream would be to establish the role played by a protein in a disease and to know the frequency at which it resonates; we could then imagine destroying this protein with an electromagnetic wave and thus blocking the entire process of disease development in a way that is non-destructive to the rest of the body. But that's still science fiction,"concludes Jérémy Torres.