Omicron, Delta, Alpha... Understanding the ball of variants
Discovered on November 25 and declared "of concern" by the WHO the following day, the Omicron variant (B.1.1.529) is being closely monitored by numerous teams. Researchers at the "Infectious Diseases and Vectors: Ecology, Genetics, Evolution and Control" unit (University of Montpellier, CNRS, IRD), Mircea Sofonea, senior lecturer, and Samuel Alizon, research director, both specialists in the epidemiology and evolution of infectious diseases, discuss the dynamics of these variants. The preponderance of Delta, the particularities of Omicron... Explanations in 10 key points from the two specialists.
Samuel Alizon, Institute of Research for Development (IRD) and Mircea T. Sofonea, University of Montpellier
The Conversation: Why did Delta overwhelm all other SARS-CoV-2 variants for months?
Samuel Alizon: The Delta variant is quite "monstrous". This can be seen, for example, in estimates of the basic reproduction number, R₀ (average number of infections caused by an infected person, in a given population). Our team estimated it at around 3 in a March 2020 report for ancestral lineages, in France. For the Alpha variant, the R₀ was between 4 and 5, which explains its rapid invasion in early 2021. For Delta, estimates are between 6 and 8.
On pourrait presque parler d’un avantage « qualitatif » sur les autres variants, comme le signalent les études de terrain : si contrôler la propagation des lignées ancestrales revenait à stopper la propagation d’une grippe pandémique (R₀ < 3), avec Delta cela s’apparente plus à contrôler un virus comme la rubéole (R₀ > 5). Ce choc est particulièrement violent pour les populations peu vaccinées ou immunisées, comme on l’a vu cet été aux Antilles ou plus récemment en Europe de l’Est.
Mircea T. Sofonea: Among human respiratory viruses, only those of mumps, chickenpox and measles are more contagious, with R₀ often estimated at more than 10. And the faster a virus spreads, the longer it will take for another variant that is only slightly more contagious to emerge.
This is a result of evolutionary biology, illustrated by Fisher's geometric model dating back to the 1930s. Schematically, each mutation can be considered as a random movement from close to close on a landscape whose relief represents the virus' ability to spread through the human population. Through natural selection, only those movements corresponding to an ascent are retained. Fisher's model suggests that this ascent takes place more and more slowly, as the probability of a random displacement falling on a higher point decreases with proximity to the top of the landscape, the adaptive optimum.
In fact, since July 2021, the number of variants has not exploded, and we have instead witnessed the diversification of the Delta variant lineage into a hundred or so sub-lineages - some of which are monitored more closely because of the mutations of interest they carry.
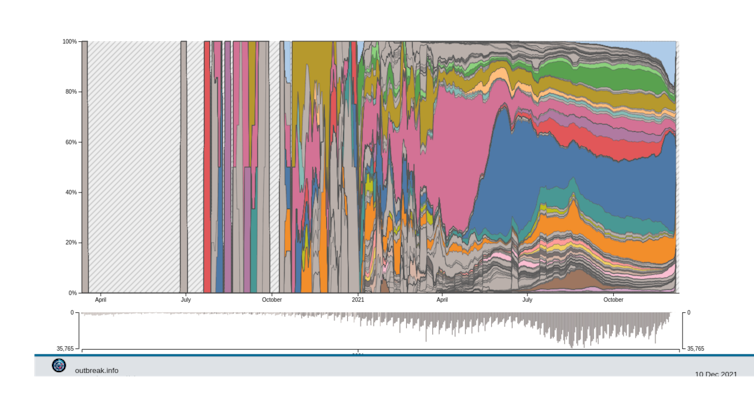
Delta Variant Report. Alaa Abdel Latif, Julia L. Mullen, Manar Alkuzweny, Ginger Tsueng, Marco Cano, Emily Haag, Jerry Zhou, Mark Zeller, Emory Hufbauer, Nate Matteson, Chunlei Wu, Kristian G. Andersen, Andrew I. Su, Karthik Gangavarapu, Laura D. Hughes
T.C.: Can Delta keep this advantage "indefinitely"?
S.A.: The more people become immune, whether through vaccination or, unfortunately, through the infections themselves, the more Delta's advantage diminishes. Indeed, we now know that other variants escape immunity better than Delta. The most common hypothesis is therefore that Delta will eventually be replaced by lines capable of infecting immune hosts. For the time being, the Beta variant is the one for which laboratory tests detect the most immune escape.
Experiments with synthetic viral proteins enable us to anticipate which mutations, or combinations of mutations, are the most important to watch out for.
T.C.: You said earlier that "we need to know how well the Delta variant has adapted to us". What is the situation?
S.A.: With twice the contagiousness of the original lines, the Delta variant is undoubtedly suited to our species in the short term. In the long term, however, it's less certain: this will depend on the duration of our immunity to the infection, and the costs associated with immune escape. Indeed, we know that certain mutations enable the virus to escape the antibodies of cured patients or vaccinated individuals... but we don't know how contagious these mutated viruses are.
M.T.S.: It's important to bear in mind that the notion of adaptation, particularly in the case of an emerging viral disease, is relative: the adaptive landscape evoked earlier is in fact animated by movements comparable to the swell. The evolution of SARS-CoV-2 is a good illustration of the "arms race" we're in with it: for the time being, we've unwillingly selected more contagious, more virulent phenotypes (Alpha, Delta variants). The focus is now on variants likely to bypass our second protective filter, immunity (post-vaccine and post-infectious).
T.C.: Where could a new variant come from?
M.T.S.: A variant appears like any other mutant, at random. Each of the nearly 30,000 bases (letters) in the SARS-CoV-2 genome mutates on average every 300,000 division cycles, and one infection can produce several billion virus particles. Ultimately, the vast majority of infected individuals can transmit different viruses from those that infected them. It is estimated that, on average, two mutations randomly attach themselves to the SARS-CoV-2 genome every month along a chain of transmission.
A particular mutant is considered a variant if it shows remarkable changes in one or more characteristics of interest (contagiousness, virulence, immune escape, symptomatology or antiviral resistance). The emergence of a variant often corresponds to a mutational jump, with evolutionary speeds 2 to 4 times higher.
In the final analysis, every infection not avoided is an opportunity for the virus to mutate and, potentially, generate a variant. Fortunately, such events are very rare, as the majority of mutations are deleterious.
It is the population in which the virus circulates that determines which mutations will be advantageous to it, in this given context (we say that selection pressures differ): if this population is not immune at all, the most contagious lineages are favored; if it is immune, then lineages capable of escaping this immunity spread more widely.
S.A.: To this we can add the case of chronic infections, particularly in immunocompromised individuals. In this case, the level of "intra-patient" selection is added to the level of population selection. It has been shown that, during an infection lasting several months, the immune system selects SARS-CoV-2 viruses with mutations that have been found in variants.
In theory, this result is not automatic, and for the human immunodeficiency virus (HIV), for example, intra-patient adaptation is thought to be to the detriment of propagation in the population. In any case, this result makes the co-circulation of HIV and SARS-CoV-2 in unvaccinated populations such as those in sub-Saharan Africa a major health issue, as has already been pointed out in connection with the evolution of the Gamma variant.
T.C.: What can you tell us about Omicron's origins?
S.A.: The new Omicron variant has been identified in South Africa, but a priori did not appear there. This country detected it thanks to the quality of its epidemiological and genomic monitoring. In this respect, the international community's rejection of this country is problematic, as it risks discouraging surveillance efforts.
While the exact origin of this variant is currently unknown, it is most likely to have originated in a region of Africa where monitoring of the epidemic is limited. Indeed, there are virtually no recent sequences of SARS-CoV-2 virus close to that of Omicron: genome analyses tell us that its common ancestor with the other variants dates back to mid-2020! This means that it could come from lines that have been circulating for over a year without being sampled (which is quite possible, given the low level of investment in monitoring the epidemic in many African countries).
It is also possible that the virus may have passed through an animal reservoir, as some of its mutations are intriguing. We know that SARS-CoV-2 can infect mammals, and in some cases, such as in mink farms, there have been cases of it returning to the human population. But for Omicron, there is still very little data to explore this hypothesis.
T.C.: The expression "evolutionary leap" has been used for certain variants. What does this mean?
S.A.: This touches on an old debate in evolutionary biology between the proponents of "gradualism", which can be traced back to Charles Darwin, and those of "punctuated equilibria" (jumps), popularized by Niles Eldredge and Stephen Jay Gould. Truth borrows from both, and the current pandemic offers a good example.
We know that SARS-CoV-2 naturally accumulates genetic mutations at a fairly regular rate, and this is what enables us to track it in phylodynamic studies. But the evolution of variants also results in widespread changes in the genome, or "selective sweeps" where a beneficial mutation (and associated sequences) becomes fixed: first with the D614G mutation(at the very start of the pandemic, the 614th amino acid of the Spike protein was usually an aspartic acid - "D" in specialized nomenclature; here it is replaced by a glycine, "G", editor's note), then with the Alpha variant (Beta and Gamma variants remained in the minority worldwide) and then the Delta variant.
So, depending on the scale you're looking at (month or year) and the criteria you're using (neutral mutations or so-called phenotypic mutations affecting biological properties such as contagiousness), you'll have a vision of continuity or jumps.
T.C.: And how do you interpret Omicron, which has 53 mutations, including some 30 in the Spike protein alone?
S.A.: For the moment, little is known about Omicron, but its mutational profile is indeed intriguing. As analyzed by South African viral evolution expert Darren Martin and his colleagues, these mutations can be classified into three groups:
- On the one hand, there are all the mutations and deletions that revolve around the N501Y mutation in the Spike protein (Δ69-70, K417N, N501Y, H655Y, P681H). The latter has already been described as profoundly altering the adaptive landscape of the virus, i.e. the field of its possibilities.
- Then, still in Spike, there is a second set of mutations at positions already mutated in other variants, for example at position 484 (N440S, S477I or E484A). These are expected to have an effect on the phenotype of infections, e.g. their contagiousness, virulence or ability to escape the immune response.
- Finally, there is a set of 14 mutations that are very rarely found in circulating lines, and even virtually absent from other known sarbecoviruses(a subgenus of betacoronaviruses that includes coronaviruses linked to severe acute respiratory syndrome, including SARS-CoV-2, editor's note). What's more, these mutations appear to be individually counter-selected. Their presence is therefore currently an enigma, as the variant seems well adapted to our species. One possibility is that there is a collective effect of these mutations, or an interaction with other mutations (such as N501Y) according to the phenomenon of epistasis, common in population genetics: even if two mutations A and B are deleterious in isolation, their joint presence may be advantageous.
It should be noted that there are already two Omicron sub-lineages. They are called BA.1 and BA.2, and do not both have the same key mutations.
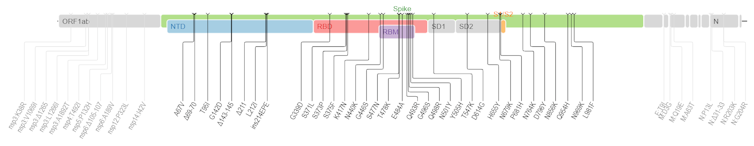
Mullen J, Tsueng G, et al, and tCfVS/https://covdb.stanford.edu/page/mutation-viewer/#omicron/Wikimedia, CC BY-SA
T.C.: Apart from these mutations, what other signals could be cause for concern?
M.T.S.: The most striking point is that Omicron is associated with a very strong epidemic resurgence in South Africa, just after the Delta variant wave. On this point, it must be said that it's very tricky to grasp national contexts. For example, our team is working hard to understand the epidemic in France. Consequently, to judge the seriousness of the epidemic situation in a country, it is best to rely on the country's epidemiologists and health agencies... And in the case of South Africa, these specialists are worried.
A number of experiments are underway in which the virus is subjected to antibodies from vaccinated or cured individuals. None has yet been published, so it's difficult to draw any conclusions, especially as preliminary results are quite divergent between studies - and even within some studies...
It should be borne in mind that these experiments indicate a general trend and do not capture the diversity of the immune response. In the end, it will be the statistical analyses of epidemiological studies that will be most useful. An initial field study suggests that the virus is capable of causing more reinfections than other strains. In other words, Omicron's rapid growth could be explained more by its ability to bypass immune responses than by its R₀.
T.C.: How widespread is Omicron worldwide?
S.A.: Omicron has already been detected at low levels in dozens of countries, including France. Such a distribution is reminiscent of what is known in scientific ecology as a "source-sink" dynamic: there is one region of the world where this virus is in the majority, and a dispersal phenomenon is underway.
In several countries with good monitoring of their epidemics, notably the UK and Denmark, we are already seeing very rapid growth in tests consistent with this variant. In fact, as it includes a deletion at position 69-70 of the Spike, one of the screening tests that includes 3 targets in the genome produces positive tests with 2 of the 3 targets present.
M.T.S.: In France, screening data, which consists in searching for specific mutations, gives us an almost real-time picture of the spread of a group of genotypes. The advantage is that this technique is less costly and faster than full genome sequencing. However, only full genome sequencing can identify a variant with certainty.
T.C.: Do you have any idea of the number of variants (of interest or of concern) that may have appeared undetected?
S.A.: Obviously, all eyes are currently on the new Omicron variant, but it's hard to say how many mutants of global interest there are. It's quite likely that several have emerged without breaking through. Indeed, even with a selective advantage, the early stages of a variant's propagation are governed by chance.
M.T.S.: In a simplistic model, the probability of extinction of an epidemic is 1/R₀. To a first approximation, with an R₀ of 3, in 33% of cases transmission chains spontaneously extinguish. Thus, several introductions of SARS-CoV-2 into France may have taken place, and the quest to identify an index case is questionable.
This is also why it is possible that there have been several emergences of variants whose transmission chains have died out on their own. In fact, super-propagation events (through unimpeded gatherings, etc.) play a key role in the spread of this virus, with the bulk of transmissions occurring in a minority of cases.
T.C.: What other variants are currently the most closely monitored?
S.A.: In France, we're keeping a close eye on the B.1.640 lineage, first detected in March 2021, notably in the Democratic Republic of Congo; it's not listed as a variant of interest by the WHO, but seems to be spreading fairly rapidly. Our team has also identified the circulation of Delta variants carrying at least two mutations associated with immune escape in the Spike protein (T95I and E484Q). For the time being, their circulation remains limited.
In any case, the emergence of the Omicron variant reminds us of the need for a long-term vision to emerge from this pandemic. Apart from the Scientific Advisory Board, few are concerned with this, as it clashes with the immediacy of political and media time. Scientific research is just one of many players with an important role to play, from developing treatments to anticipating viral evolution.
Unfortunately, it is a long-term process, which makes it unattractive to politicians... and it bears the brunt of a scientific inculture that permeates almost all sectors of French society. With the recent abolition of biology and physics/chemistry classes for the majority of high school students, the belief in miraculous solutions is likely to get even worse.![]()
Samuel Alizon, Director of Research at CNRS, Institute of Research for Development (IRD) and Mircea T. Sofonea, Senior Lecturer in Epidemiology and Evolution of Infectious Diseases, MIVEGEC Laboratory, University of Montpellier
This article is republished from The Conversation under a Creative Commons license. Read theoriginal article.