[LUM#2] Tomorrow, healing
Targeted, sustainable medicine that no longer merely relieves symptoms but offers the luxury of repair. Thanks to advances in stem cell research, regenerative medicine is set to revolutionize the medical world.
Is osteoarthritis causing you pain? Get back the knees you had in your twenties with brand new cartilage. Is Alzheimer's looming? Replace your failing neurons with more efficient ones. Is your heart threatening to give out? Replace it! Science fiction? No, the promises of regenerative medicine. Its goal: "to repair an injury or diseased organ by replacing failing cells with healthy ones ," explains Christian Jorgensen. This targeted medicine makes it possible to consider completely curing certain diseases rather than simply treating the symptoms with heavy medication.
The fabulous potential of stem cells
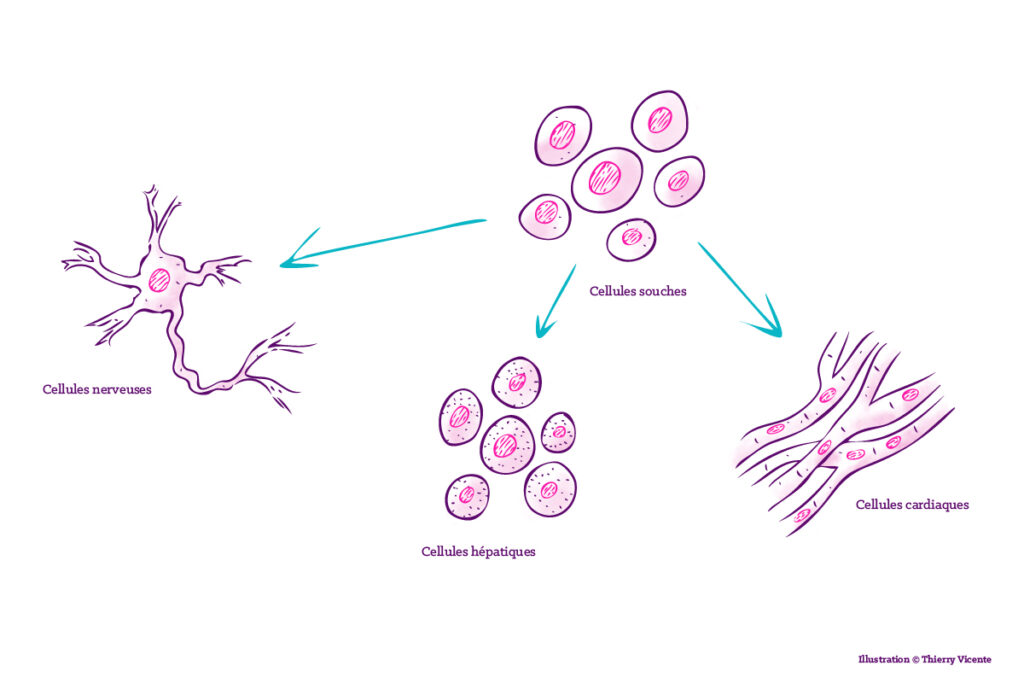
In Montpellier, this medicine of the future is already a reality: researchers atthe Institute for Regenerative Medicine and Biotherapy (IRBM) are at the forefront of this discipline. Their main material? Stem cells. These cells have the unique ability to give rise to different cell types, enabling the production of cartilage, neurons, heart tissue, liver tissue, skin, and more.
There are two types: multipotent stem cells, which can differentiate into a limited number of cell types, and pluripotent stem cells, which can give rise to all the cells in the body (see box). How? "Thanks to a cocktail of molecules that induces their differentiation into a specific tissue,"explains the director of the IRMB. A "liver" cocktail to obtain liver cells, a "heart" cocktail to obtain cardiomyocytes, whichever you choose. The advantage of this technique is that cells can be taken from the patient themselves and re-injected after differentiation, without the risk of the immune system rejecting the graft.
Numerous clinical trials are underway to test these new therapies. In Montpellier, researchers are treating patients suffering from osteoarthritis of the knee as part of the European Adipoa clinical trial led by Christian Jorgensen. "We use mesenchymal stem cells, a type of multipotent cell found throughout the body," explains the researcher. These cells are injected directly into the patients' knees to regenerate damaged cartilage. An initial trial involving 18 patients yielded very encouraging results: 80% of them reported improved functionality and reduced pain within nine months of the injection. " We have now launched a second, larger-scale trial involving 150 patients, with results expected in late 2018."
Create organs
Osteoarthritis, heart attacks, heart failure, macular degeneration, Alzheimer's and Parkinson's diseases... The list of diseases that can be targeted by stem cells continues to grow. But regenerative medicine has even greater ambitions. The goal: to create organs. "We know how to produce heart cells. We also know how to reconstruct the matrix of a human heart from collagen fibers using a 3D printer. By using these heart cells to 'colonize' this matrix, we can create a human heart, an organoid that can be transplanted into a patient without any risk of rejection," explains Christian Jorgensen, who believes that this dream could become a reality within five years... perhaps in Montpellier. "We have all the infrastructure we need to be at the forefront of regenerative medicine thanks to a platform that brings together medicine, robotics, chemistry, and imaging. Montpellier is a leader in this field," says the director of the IRMB. The goal is to meet a major challenge facing our society: living longer, but above all, living healthier lives.
Researchers hit "reset"
Access to pluripotent stem cells has long been limited, as they are only found naturally in embryos. Now, researchers are able to manufacture them. How do they do it? They take a small skin sample from a patient and extract cells from it, which they then genetically "reprogram." "It's like pressing a 'reset' button to reset the cell," explains Christian Jorgensen. This produces "induced pluripotent stem cells" or IPS cells, which can be transformed into any type of cell. This technique earned its inventor, Japanese researcher Shinya Yamanaka, the 2012 Nobel Prize in Medicine.
Find UM podcasts now available on your favorite platform (Spotify, Deezer, Apple Podcasts, Amazon Music, etc.).